Manténgase sano!
- Dennis Thompson
- Posted June 16, 2025
Innovative Once-Weekly Capsule Helps Quell Schizophrenia Symptoms
A new breakthrough can help people with schizophrenia keep up with their psychiatric meds, researchers said.
A pill taken just once a week, gradually releasing medicine from within the stomach, can greatly simplify the drug schedule faced by schizophrenia patients, researchers reported June 10 in the journal The Lancet.
The newly developed pill maintains consistent levels of the psychiatric drug risperidone in patient’s bodies, and controlled their symptoms just as well as daily doses, results showed.
“We’ve converted something that has to be taken once a day to once a week, orally, using a technology that can be adapted for a variety of medications,” researcher Giovanni Traverso, an associate professor of mechanical engineering at MIT, said in a news release.
“The ability to provide a sustained level of drug for a prolonged period, in an easy-to-administer system, makes it easier to ensure patients are receiving their medication,” he said.
These final-stage clinical trial results are the product of more than 10 years of research by Traverso’s lab.
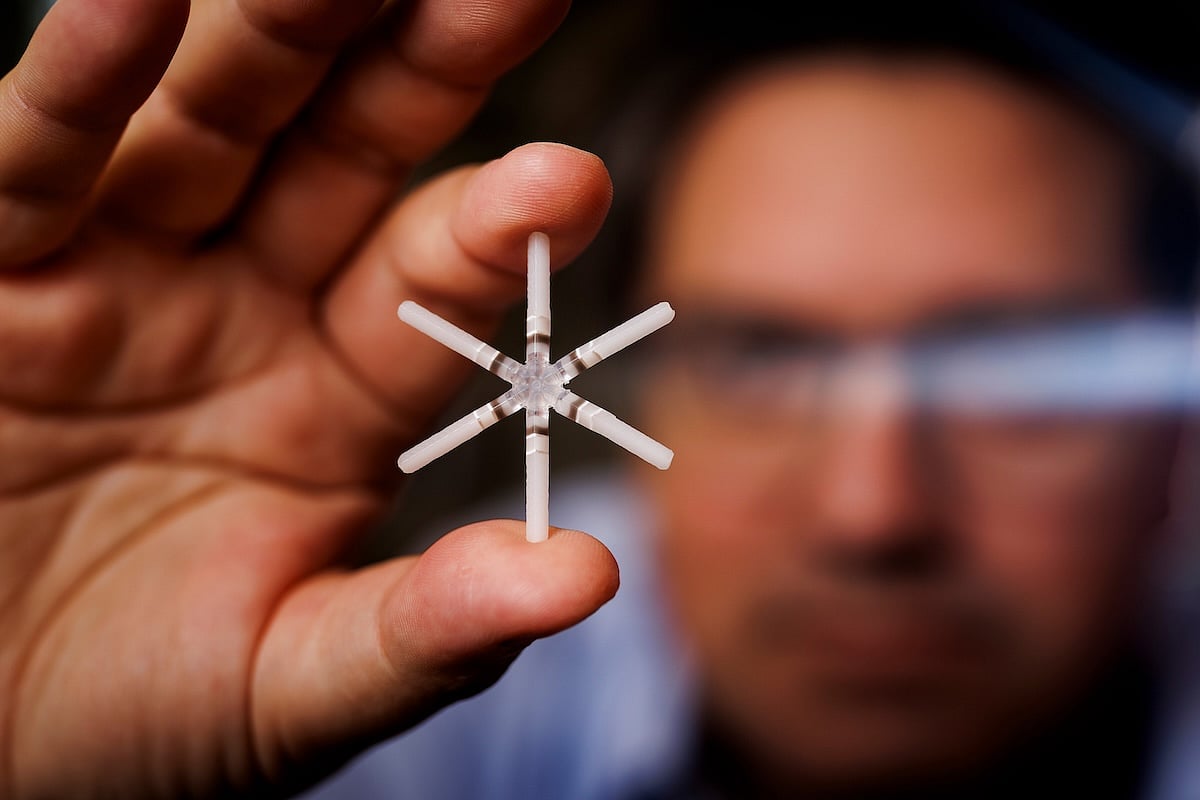
The capsule, about the size of a multivitamin, expands into a six-armed star shape that helps it remain in the stomach until all the drug is released, researchers said.
Once the arms are extended, the device becomes too large to pass through the exit of the stomach.
It floats freely in the stomach and slowly releases drugs from the arms for about a week, researchers said. The dissolving arms eventually break off, allowing the pill to pass through the digestive tract.
Traverso’s lab reported the development of the star-shaped drug device in 2016, and then teamed with pharmaceutical company Lyndra Therapeutics to test its use with a specific drug.
The chosen drug, risperidone, is commonly prescribed to treat schizophrenia. Most patients take the drug once a day, researchers noted.
“One of the areas of unmet need that was recognized early on is neuropsychiatric conditions, where the illness can limit or impair one’s ability to remember to take their medication,” Traverso said. “With that in mind, one of the conditions that has been a big focus has been schizophrenia.”
For the clinical trial, researchers recruited 83 schizophrenia patients at five sites across the U.S.
Of those patients, 47 completed the full five weeks of the study, in which they took one risperidone-loaded capsule per week, the paper says.
Researchers measured the amount of drug in each patient’s bloodstream. Each week, they observed a sharp increase on the day the patient took the capsule, followed by a slow decline over the next week.
Levels all fell within the optimal range, and varied less over time than what occurs when patients are taking a daily risperidone pill, researchers said.
Patients’ symptoms also remained stable throughout the study, researchers noted.
“One of the biggest obstacles in the care of people with chronic illnesses in general is that medications are not taken consistently. This leads to worsening symptoms, and in the case of schizophrenia, potential relapse and hospitalization,” said lead researcher Dr. Leslie Citrome, a clinical professor of psychiatry and behavioral sciences at New York Medical College.
“Having the option to take medication by mouth once a week represents an important option that can assist with adherence for the many patients who would prefer oral medications versus injectable formulations,” he added in a news release.
Side effects from the time-release capsule were minimal, with some patients experiencing short-term mild acid reflux and constipation, researchers said.
“This really demonstrates that what we had hypothesized a decade ago, which is that a single capsule providing a drug depot within the GI tract could be possible,” Traverso said. “Here what you see is that the capsule can achieve the drug levels that were predicted, and also control symptoms in a sizeable cohort of patients with schizophrenia.”
However, researchers plan to perform larger phase 3 studies before applying for U.S. Food and Drug Administration approval of this delivery approach for risperidone. They also plan to start early-phase clinical trials exploring the use of the star-shaped device to deliver other types of drugs, including contraceptives.
Lyndra Therapeutics funded this clinical trial.
More information
The National Alliance on Mental Illness has more on risperidone.
SOURCE: MIT, news release, June 10, 2025





